Conflict and Cans: "Periodic Table and Trends" Unit (PBL) for HS Chemistry
- Zip
What educators are saying
Also included in
- This BUNDLE consists of a year's worth of Chemistry Problem-Based Learning Units. They are also sold separately on TPT.All of the units have two sets of files. The "Classroom" files should be used in an in-person classroom setting. The "Absent" files can be used for long-term distance learning, homePrice $76.10Original Price $85.80Save $9.70
Description
Conflict and Cans: A Problem-Based "Periodic Table and Trends" Unit (PBL) for High School Chemistry
Summary:
Your company, Can-Do Enterprises, is a manufacturer of tin cans, steel cans with a tin-plating. Recently, one of your tin suppliers has been accused of trading in conflict resources. To avoid bad press (and bad karma), Can-Do is looking into the feasibility of changing its plating from tin to another elemental material.
See the preview for the complete introduction, a list of materials, and the first day activities.
Each file includes a classroom version of the assignment and an absent/ remote version. The absent/ remote version may require internet access but does not require any other supplies.
This product is also part of a BUNDLE found here.
Previous knowledge: physical and chemical properties, states of matter, atomic theory, basic atom anatomy
Objectives:
General:
- Construct models using Bohr's nuclear atom.
- Describe the structure of atoms and ions, including the masses, electrical charges, and locations of protons and neutrons in the nucleus and electrons in the electron cloud.
- Construct models to express the arrangement of electrons in atoms of representative elements using electron configurations and Lewis dot structures.
- Explain the development of the Periodic Table over time using evidence such as chemical and physical properties.
- Predict the properties of elements in chemical families, including alkali metals, alkaline earth metals, halogens, noble gases, and transition metals, based on valence electrons patterns using the Periodic Table.
- Analyze and interpret elemental data, including atomic radius, atomic mass, electronegativity, ionization energy, and reactivity to identify periodic trends.
NGSS:
HS-PS1-1. Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms.
HS-PS2-6. Communicate scientific and technical information about why the molecular-level structure is important in the functioning of designed materials.
HS-PS1-2. Construct and revise an explanation for the outcome of a simple chemical reaction based on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.
HS-ETS1-1. Analyze a major global challenge to specify qualitative and quantitative criteria and constraints for solutions that account for societal needs and wants.
HS-ETS1-2. Design a solution to a complex real-world problem by breaking it down into smaller, more manageable problems that can be solved through engineering.
HS-ETS1-3. Evaluate a solution to a complex real-world problem based on prioritized criteria and trade-offs that account for a range of constraints, including cost, safety, reliability, and aesthetics as well as possible social, cultural, and environmental impacts.
Contents:
Day 1:
- Introduction
- Getting to Know the Can: Cans come in many different shapes, sizes, and materials. What are the advantages and disadvantages to different types of packaging?
Day 2:
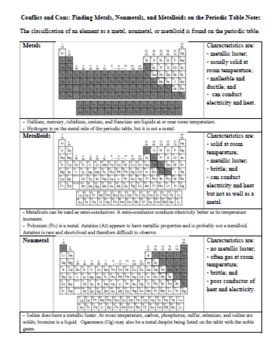
- Metals, Nonmetals, and Metalloids Lab: Hand-on. What are the physical properties of five unknown element samples? Can these properties be used to classify the samples as metals, nonmetals, or metalloids?
- Finding Metals, Nonmetals, and Metalloids on the Periodic Table Notes: How does the periodic table allow us to predict the physical properties of elements?
- Metals, Nonmetals, and Metalloids on the Periodic Table Practice: Practice
Day 3:
- Groups and Periods: How can the periodic table be used to predict the number of energy levels and valence electrons in an atom?
- Element Joke Activity: Practice
Day 4:
- Lewis Dot Structures: How do you draw a Lewis dot structure?
- Quantum Mechanical Model Introduction: How is the quantum mechanical model different from the Bohr model?
- Orbital Modeling Activity: Hands-on. What to the orbitals look like in the quantum mechanical model? How do they all fit together and hold electrons?
Day 5:
- Shady Acres Home for Grumpy Electrons Simulation: Hands-on. Why do electrons fill the energy levels and orbitals in the order that they do?
- Orbital Diagram Practice: Practice
Day 6:
- Electron Configurations: How do you write an electron configuration from an orbital diagram?
- Electron Configurations Practice: Practice
Day 7:
- Standard and Noble Gas Configurations: How else can you write an electron configuration?
- Electron Configurations Battleship: Game. Practice
Day 8:
- Introducing Patterns on the Periodic Table: What patterns have we already seen?
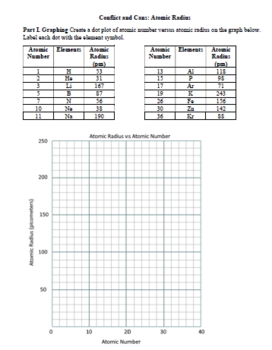
- Atomic Radius: Data analysis. What factors determine the radius size of an atom? How is this trend predicted by the periodic table?
Day 9:
- Ionization Energy: Data analysis and lab. What is ionization energy?How does ionization energy predict reactivity of alkali metals and alkaline earth metals?
Day 10:
- Ionization Energy Continued: Data analysis and lab (or demo). What does ionization energy tell us about the transition metals?
- Ionic Radius of Positive Ions: Can the trends for ionic radius of positive ions be predicted?
Day 11:
- Electronegativity: What is electronegativity? How does it predict reactivity?
- Ionic Radius of Negative Ions: Can the trends for ionic radius of negative ions be predicted?
Day 12:
- Summarizing Notes: Students use their work to summarize their learning.
- Table and Trend Practice: Students practice/ review using the trends.
Days 13 – 15:
- Research and Report
Day 16:
- Test (not included)
Copyright © E. Stubbe (The Wasp Whisperer)
All rights reserved by author.
Terms of Use: This document is for personal use only and may only be used by the original purchaser. This entire document, or any parts within, may not be reproduced or displayed for public viewing. You may NOT electronically post this product online including to teacher blogs, classroom websites or school networks. Failure to comply is a copyright infringement and a violation of the Digital Millennium Copyright Act (DMCA).