Chemistry Real Life Stoichiometry Problems KEY included gram to gram and mole
- PDF
- Easel Activity
What educators are saying
Description
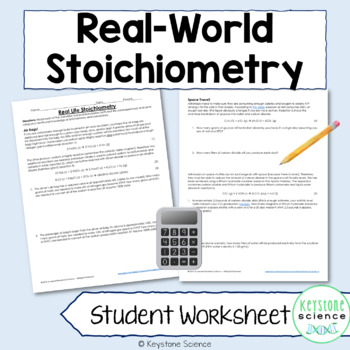
Students sometimes struggle applying what they are learning in science to the real world. I developed these “Real Life Stoichiometry Problems” to help students see how these types of problems are used in the “real world”. The examples on this worksheet detail the stoichiometry involved in air bags and space travel. These problems are best for students that have mastered all aspects of stoichiometry problems.
You will receive an uneditable PDF file with a two-page student handout and 3 page teacher key. The answer key not only includes how each problem is set up but a written explanation for how to solve the problem.
Related Products
⭐ Crash Course Stoichiometry Guided Notes with KEY Digital Learning
⭐ FREE Steps to Solve Stoichiometry Problems Chemistry Reference Page
⭐ Chemical Reactions and Equations PowerPoint with Guided Notes
*NEW* products are added periodically! ;)
Customer Tips
How to get TPT credit to use on future purchases
Please go to your My Purchases page (you may need to login). Beside each purchase you'll see a Provide Feedback button. Simply click it and you will be taken to a page where you can give a quick rating and leave a short comment for the product. Each time you give feedback, TPT gives you feedback credits that you use to lower the cost of your future purchases. I value your feedback greatly as it helps me determine which products are most valuable for your classroom so I can create more for you. ☺
Be the first to know about my new discounts, freebies and product launches
Look for the green star next to my store logo and click it to become a follower. Voila! You will now receive email updates about this store. ☺