Atoms and Periodic Table Bohr Model Diagrams MS-PS1-1
- Zip
- Internet Activities
What educators are saying
Also included in
- This NGSS bundle has everything you need for Structure and Properties of Matter (complete distance learning unit), PS1.B PS3.A Chemical Reactions Google Ready Bundle (complete distance learning unit) Chemical Reactions, Definitions of Energy, States of Matter, Periodic Table, Chemical Reactions, ConPrice $69.97Original Price $115.20Save $45.23
- This bundle is a time-saving, comprehensive and user-friendly complete year bundle for physical science Teachers that will streamline your teaching experience effortlessly! Every lesson is meticulously planned and just a click away. This extraordinary bundle is a treasure trove of engaging resourcesPrice $176.00Original Price $311.50Save $135.50
- The TEKS 8th Grade Bundle is a comprehensive resource for teaching science mainly in Texas, but it can be used in any state. This bundle includes over 60 hands-on, rigorous, engaging, and phenomena-driven lessons that cover the TEKS standards for 8th grade science. The lessons are designed to help sPrice $160.00Original Price $321.15Save $161.15
Description
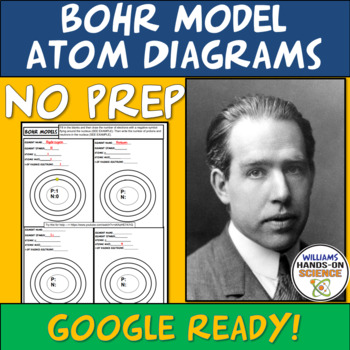
These worksheets are no prep and easy for students to understand once you have provided the scaffolding to do Bohr Model Atom Diagrams. Use it for HW, classwork, distance learning or sub-work. Easy to use key is provided.
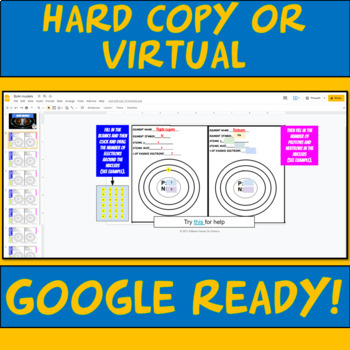
Google Ready for at home or in person options.
These concepts are included:
-Element name
-Element symbol
-Atomic mass
-Number of Protons, neutrons and electrons
-Number of electrons on each ring
-Number of valence electrons
-Periodic Table
This Activity covers NGSS Standard:
PS1.A: Structure and Properties of Matter
You get:
-Hard copy of worksheet
-Hard copy of the key
-Google Ready instructions for doing the assignment on the computer.
TERMS OF USE
• All rights reserved by Williams Hands On Science, Inc.
• This product is to be used by the original purchaser only.
• Intended for classroom and personal use only.
• Copying for more than one teacher, classroom, department, school, or school system is prohibited.
• This product may not be distributed or displayed digitally for public view.
• Failure to comply is a copyright infringement and a violation of the Digital Millennium Copyright Act (DMCA).
If there are any errors or questions, please contact me through TpT or email me at:
williamshandsonscience@gmail.com
Thank you for taking a look!
Please follow me on TpT for new products and check me out on Instagram for my products in action!
https://www.instagram.com/williams_hands_on_science/
Or buy my bundle that includes all of CHEMISTRY products: ngss Chemistry Bundle
Elements, Atoms, Compounds and Molecules Card Sort
Flipping the Classroom Periodic Table Frayer Models
Physical Science Scaffolded Notes
Check out my bundle that includes all of my physics products NGSS Physics Resource Bundle