Arson or Accident: Chemical Reactions, Stoichiometry, and Heat Unit (Storylined)
- Zip
What educators are saying
Also included in
- This BUNDLE consists of a year's worth of Chemistry Problem-Based Learning Units. They are also sold separately on TPT.All of the units have two sets of files. The "Classroom" files should be used in an in-person classroom setting. The "Absent" files can be used for long-term distance learning, homePrice $76.10Original Price $85.80Save $9.70
Description
Arson or Accident? A Problem-based "Chemical Reactions, Stoichiometry, and Heat" Unit for High School Chemistry
Story: Cameron Todd Willingham was put on trial for a 1991 house fire that killed his three children. He was found guilty and executed in 2004. While the evidence and testimony presented at his trial seemed to point toward his guilt, as the date of his execution grew near, it became clear that the evidence his conviction was based on was not scientifically sound. Students will use principles of conservation or matter, conservation of energy, thermochemistry, and introductory stoichiometry to evaluate the trial evidence.
Please see the preview for a list of materials and the first day activities.
This product is also part of a BUNDLE found here.
An absent version of each lesson is included.
Objectives:
- Interpret, write, and balance chemical equations, including synthesis, decomposition, single replacement, double replacement, and combustion reactions using the law of conservation of mass.
- Perform stoichiometric calculations, including determination of mass relationships and percent yield.
- Describe the concept of limiting reactants in a balanced chemical equation.
- Investigate the process of heat transfer using calorimetry;
- Classify processes as exothermic or endothermic and represent energy changes that occur in chemical reactions using thermochemical equations or graphical analysis.
- Perform calculations involving heat, mass, temperature change, and specific heat.
NGSS Objectives:
-HS-PS1-4. Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends on total bond energy.
-HS-PS1-7. Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
Day-by-Day
The case is divided into 3 parts to allow for testing on each section before moving on.
Part I: The Fire - Chemical Equations and Conservation of Matter
Day 1:
-Introduction - Reading and Videos
Day 2:
-Types of Reactions - Lab
Day 3:
- Counting Atoms - Worksheet
- Modeling Combustion Reactions - Hands-on activity
Day 4:
-Balancing Equations Introduction - Computer activity
- Willingham's Statement - Reading
Day 5:
-Practice with Balancing Equations - Worksheet and rotation activity
Day 6:
-Predicting Products 1 - Worksheet
-More Witnesses - Reading
Day 7:
-Predicting Products 2 - Worksheet
-Even More Witnesses - Reading
Optional Day or 2:
- Optional review or quiz day (resources not included)
Part II: The Investigation - Stoichiometry
Day 8:
- Investigating the Origin - Reading
- Introduction to Stoichiometry - Worksheet
Day 9:
-How Materials React Part I - Lab
Day 10:
- How Materials React Part II - Lab
Day 11:
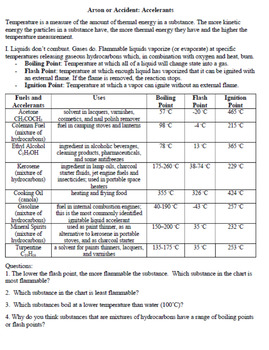
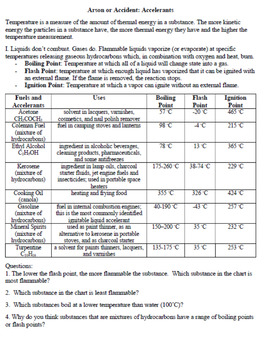
- Accelerants and Limiting Reactants - Worksheet
Day 12:
- Making a Case - Reading and writing
Optional Day or 2:
- Optional review or quiz day (resources not included)
Part III: Heat - The Truth?
Day 13:
- Heat and Calorimetry - Lab
Day 14:
- Energy in Bonds - Lab
Day 15:
- Flashover - Worksheet
Day 16:
- Execution
Optional Day or 2:
- Optional review or quiz day (resources not included)
Copyright © E. Stubbe (The Wasp Whisperer)
All rights reserved by author.
Terms of Use: This document is for personal use only and may only be used by the original purchaser. This entire document, or any parts within, may not be reproduced or displayed for public viewing. You may NOT electronically post this product online including to teacher blogs, classroom websites or school networks. Failure to comply is a copyright infringement and a violation of the Digital Millennium Copyright Act (DMCA).