An Aquatic Apocalypse – "Solutions Chemistry" Unit (PBL)
- Zip
What educators are saying
Also included in
- This BUNDLE consists of a year's worth of Chemistry Problem-Based Learning Units. They are also sold separately on TPT.All of the units have two sets of files. The "Classroom" files should be used in an in-person classroom setting. The "Absent" files can be used for long-term distance learning, homePrice $76.10Original Price $85.80Save $9.70
Description
An Aquatic Apocalypse: A Problem-based "Solutions Chemistry" Unit (PBL) for High School Chemistry
Summary:
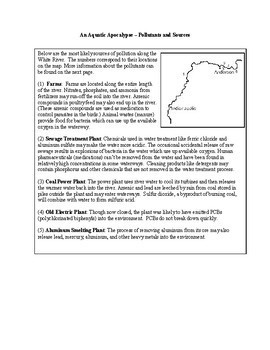
Millions of fish are dead along a stretch of the White River that passes through Indiana. So, what happened? This problem-based unit asks students to complete a series of experiments to determine the possible cause of the fish kill.
See the Preview for a list of materials and other background information as well as the first day activities.
Each day has an "in-class" and "absent" version of the assignment.
This product is also part of a BUNDLE found here.
Previous knowledge: Students should have already learned stoichiometry.
Objectives:
This problem-based unit was designed to teach the required objectives for solution chemistry in the state of Texas. It would likely work with or without modification in many other venues.
- Interpret, write, and balance chemical equations, including synthesis, decomposition, single replacement, double replacement, and combustion reactions using the law of conservation of mass.
- Differentiate among acid-base reactions, precipitation reactions, and oxidation-reduction reactions.
- Describe the unique role of water in solutions in terms of polarity.
- Distinguish among types of solutions, including electrolytes and nonelectrolytes and unsaturated, saturated, and supersaturated solutions.
- Investigate how solid and gas solubilities are influenced by temperature using solubility curves and how rates of dissolution are influenced by temperature, agitation, and surface area.
- Investigate the general rules regarding solubility and predict the solubility of the products of a double replacement reaction.
- Calculate the concentration of solutions in units of molarity.
- Calculate the dilutions of solutions using molarity.
- Name and write the chemical formulas for acids and bases using IUPAC nomenclature rules.
- Define acids and bases and distinguish between Arrhenius and Bronsted-Lowry definitions.
- Differentiate between strong and weak acids and bases.
- Predict products in acid-base reactions that form water.
- Define pH and calculate the pH of a solution using the hydrogen ion concentration.
NGSS: HS-PS1-2, HS-PS1-5, HS-PS1-7, HS-ESS2-5
Contents:
Day 1:
- Introduction and Solution Vocabulary (lab)
- Pollutants
Day 2:
- Solubility Notes
- Solubility Rules (lab)
Day 3:
- Concentration
- Solubility Rules WS
Day 4:
- Dilutions (lab)
- Practice with Molarity WS
Day 5:
- Solubility vs Temperature (lab)
Day 6:
- River Data
- Solubility Graphs WS
Day 7:
- Solubility of Gases (lab)
- More Solubility Graphs WS
Day 8:
- Acids and Bases Introduction (lab)
Day 9:
- Naming Acids and Bases
- Acids as Pollutants
Day 10:
- Calculating pH
- Strong and Weak Acids and Bases (lab)
- Summary
Day 11:
- Neutralization (lab)
- Report
Copyright © E. Stubbe (The Wasp Whisperer)
All rights reserved by author.
Terms of Use: This document is for personal use only and may only be used by the original purchaser. This entire document, or any parts within, may not be reproduced or displayed for public viewing. You may NOT electronically post this product online including to teacher blogs, classroom websites or school networks. Failure to comply is a copyright infringement and a violation of the Digital Millennium Copyright Act (DMCA).